Abstract
Emerging evidence indicates that the loss of intestinal microbiota diversity affects the graft-versus-host disease (GVHD) severity and transplant-related mortality. Recently, it has been speculated that oral dysbiosis in allogeneic hematopoietic cell transplantation (HCT) patients may be associated with acute GVHD; however, the mechanism by which periodontal inflammation induces the development of complications after HCT, especially GVHD, has not been fully elucidated. Oral inflammatory diseases, such as periodontitis, exacerbate systemic inflammation by exposing the target organs to both oral pathobiont microbiota and pathogenic T cells. Herein, we focused on the possibility of an association between oral mucosal inflammation and GVHD.
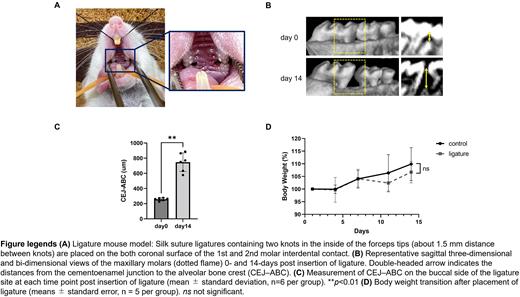
To determine the effect of oral dysbiosis on GVHD, we used a ligature-induced periodontitis model (Figure A). Ligature placement induced osteoclast infiltration based on tartrate resistant acid phosphatase staining and alveolar bone loss as observed on three-dimensional-computed tomography (3D-CT) (Figure B-C). The mice in the ligature group did not experience weight loss post-insertion (Figure D) nor did they show inflammatory cell infiltration in the major organs on histological images when compared to the control mice. On day 14 after oral ligation, these mice underwent HCT in the miHA-mismatched B10.D2 (H-2d) → BALB/c (H-2d) model of chronic GVHD. The recipient BALB/c mice were sublethally irradiated (5.5 Gy) and transplanted with 5×106 bone marrow and 2×106 splenic T cells from either syngeneic BALB/c or allogeneic B10.D2 donors. GVHD scores 3 weeks after HCT, especially the skin injury score, increased significantly in the allogenic ligature mice (p<0.05), but the overall survival (OS) decreased in them (p=0.03 compared with allogeneic control mice). Histopathological examination of the target organs 3 weeks after HCT confirmed severe GVHD in the ligature mice (p<0.05). Moreover, the absolute number of IFN-γ+CD8+ and IL-17+CD4+ cells in the lymph nodes of the allogeneic ligature mice increased significantly (p<0.05). To avoid strain-specific effects, we used another chronic GVHD model, C57BL/6 (H-2b) → B6D2F1 (H-2b/d), which showed similar results of increased GVHD scores and shorter OS in the allogenic ligature mice.
To investigate the mechanism of chronic GVHD exacerbation in oral dysbiosis cases, we analyzed the cervical lymph nodes before HCT. The absolute number of dendritic cells (DCs) from the cervical lymph nodes increased significantly in the ligature mice as compared to that in the control mice, as did the activated DC markers such as MHC class II, CD40, CD80, CD86, PD-L1, and PD-L2 (p<0.05).
To characterize the effects of oral dysbiosis on chronic GVHD progression further, we removed the ligature. The allogeneic mice that underwent ligature removal on day 0 after HCT tended to show an improvement in the OS (p=0.08) and GVHD scores as compared to those that did not undergo ligature removal. Furthermore, the application of an oral antibiotic ointment (combination of vancomycin, minocycline, clindamycin, metronidazole, and ciprofloxacin) to improve oral dysbiosis ameliorated the alveolar bone loss observed on 3D-CT in the ligature mice without HCT. Additionally, the GVHD scores improved significantly in the allogeneic ligature mice receiving oral antibiotic ointment treatment from day 0 to day 35 after HCT as compared to those receiving vehicle treatment (p<0.05). Allogeneic ligature mice receiving oral antibiotic ointment treatment showed significantly better OS than those not receiving it (p<0.05).
Our data demonstrated that oral dysbiosis caused chronic GVHD exacerbation. The mechanism of GVHD exacerbation could be related to T-cell activation by antigen-presenting cells in the cervical lymph nodes stimulated by ligature-induced oral inflammation. This study demonstrates that treating oral inflammation that causes oral dysbiosis could induce immunological tolerance and could be the therapeutic target in chronic GVHD treatment.
Disclosures
Asada:Nippon Shinyaku: Speakers Bureau; Meiji: Speakers Bureau; Kyowa KIRIN: Speakers Bureau; Astellas: Speakers Bureau; Asahi KASEI: Speakers Bureau; Abbvie: Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Ennishi:Eisai Pharmaceutical Co., Ltd.,: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Kyowa Kirin Pharmaceutical Co., Ltd.: Honoraria; Nipponshinyaku Pharmaceutical Co., Ltd.,: Research Funding. Maeda:Eisai: Honoraria; Kyowa Kirin: Honoraria; Takeda: Honoraria; AstraZeneca: Research Funding; Astellas: Honoraria, Research Funding; Chugai Pharmaceutical: Honoraria, Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Novartis Pharma: Research Funding, Speakers Bureau; Otsuka Pharmaceutical: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal